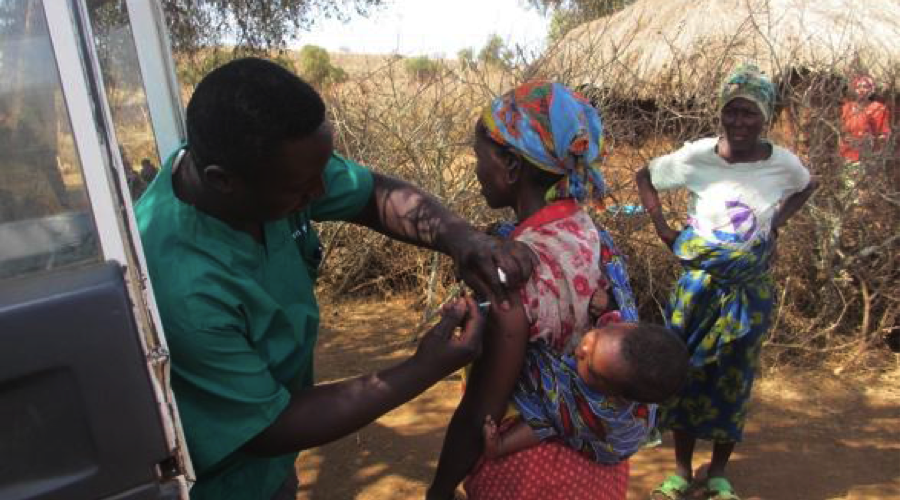
C-Fam Exclusive: UNICEF Denies Giving Kenyan Women Birth Control Vaccine Without Their Knowledge
This is an exclusive interview of C-Fam with James Elder, UNICEF’s Chief of Communications for East Africa. The interview follows allegations of the Kenya Catholic Bishops Conference and the Kenya Catholic Doctors Association that vials of tetanus vaccine distributed in a special neo-natal program in Kenya tested positively for hCG in 4 unrelated laboratories. hCG is a hormone that has been used in research and development of fertility regulating vaccines, and has been allegedly given to women without their knowledge in vaccination programs during the 1990s. The hormone inhibits the natural production of hCG which is necessary for a newly formed embryo to implant in the uterus, and could cause multiple miscarriages and even infertility.
C-Fam: Are WHO and UNICEF experimenting with hCG vaccines in Kenya?
Elder: No.
The aim of the vaccination is to eliminate neonatal tetanus in Kenya through vaccination campaigns in districts that have been identified as at high risk for maternal and neonatal tetanus.
C-Fam: Why would only the female population of child bearing age be targeted for this specific tetanus vaccination program?
Elder: The aim of the vaccination campaign is to prevent neonatal tetanus in newborns who are the most vulnerable and bear the highest burden of tetanus disease. These children live in the parts of the country with limited access to health facilities — most births occur at home in unhygienic conditions. Some of the babies are born to girls as young as 15 years old from communities living in marginalized areas. Children born at home under unhygienic conditions are at risk of tetanus infection through the cut umbilical cord. Their mothers are also at risk of infection with tetanus infection during childbirth.
Vaccinating girls and women of child bearing age (15 to 49 years) accords protection to the women even if they deliver at home in unhygienic conditions. They pass this protection to the unborn child in the womb. For the babies born to women who have received the required doses of the vaccine this protection from tetanus lasts for a few weeks after birth. That is why they have to get TT vaccine again through the routine immunization programme.
C-Fam: Why does the Tetanus vaccine require 5 doses, when usually tetanus vaccination only requires one shot every 5 to 10 years?
Elder: During vaccination campaigns that aim to protect newborns living in areas with limited access to health facilities, 3 doses are administered. The second dose after 1 month or soon thereafter and the third dose after 6 months. The 3 doses provide protection for 5 years. These are additional doses as most people have received some TT vaccine when they cut themselves or during visits to Antenatal clinics when pregnant. Five doses are recommended in the Kenya Vaccination policy to anyone (male or female) as it offers protection against tetanus for life.
C-Fam: Have you seen the test results of the tetanus vaccine from the Kenya Catholic Doctors Association which found the tetanus vaccine laced with hCG?
Elder: Yes. The tests were done in hospital laboratories in Kenya. The staff in these laboratories could not however tell whether the samples were vaccines or not, as this was not declared to the testing laboratories by the Catholic Doctors Association. The laboratories tested the samples for hCG using analyzers used for testing human samples like blood and urine for pregnancy. There is no laboratory in Kenya with the capacity to test non-human samples like vaccine for hCG. PLS see statement for more details.
C-Fam: Why is UNICEF taking so long to say anything about this?
Elder: The Kenya Ministry of Health has been taking the lead in responding. UNICEF has been working with the ministry to address this issue through media interviews and dialogue with the Catholic doctors. We also hoped the Catholic Church would do due diligence before making such allegations.
Several meetings were held between the Ministry and the church before, during and after the campaign. We also provided the relevant documents to facilitate discussions on this issue.
C-Fam: What are the respective roles of WHO, UNICEF, and the Kenya Government in the planning and the execution of this program?
Elder: The Government of Kenya through the Ministry of Health is responsible for the overall leadership and coordination of the program because this is a program that is aimed at addressing the needs of its people. They guide the process, and provide the relevant health data needed to identify the districts to be reached. The Government links all stakeholders to ensure the success of the program.
WHO provides technical support and guidance to the Government in pre-qualifying vaccine manufacturers, ensures that the vaccines manufactured meet the required standards, monitors any vaccine-related adverse outcomes following immunization, data analysis, monitoring of the program and ensures quality of the campaign.
UNICEF provides technical support and guidance in communication, advocacy and social mobilization of the communities and stakeholders to ensure the vaccination reaches all those targeted. UNICEF provides the funds to buy the vaccines from WHO-prequalified manufacturers, delivery of the vaccines to the country and health facilities. In addition, UNICEF supports health workers to travel to all parts of the districts to give the vaccines to those targeted for vaccination and to meet their supervisors to monitor the quality of the campaign.
C-Fam: Who manufactured the Vaccines?
Elder: The vaccines were sourced from the Serum Institute of India. A WHO pre-qualified manufacturer.
C-Fam: What role if any did UNICEF have in the selection of this specific vaccine for the program in Kenya?
Elder: UNICEF contracts WHO pre-qualified manufacturers to supply vaccines to meet the needs of countries. This is through a competitive and an open tender process.
View online at: https://c-fam.org/turtle_bay/c-fam-exclusive-unicef-denies-giving-kenyan-women-birth-control-vaccine-without-knowledge/
© 2024 C-Fam (Center for Family & Human Rights).
Permission granted for unlimited use. Credit required.
www.c-fam.org