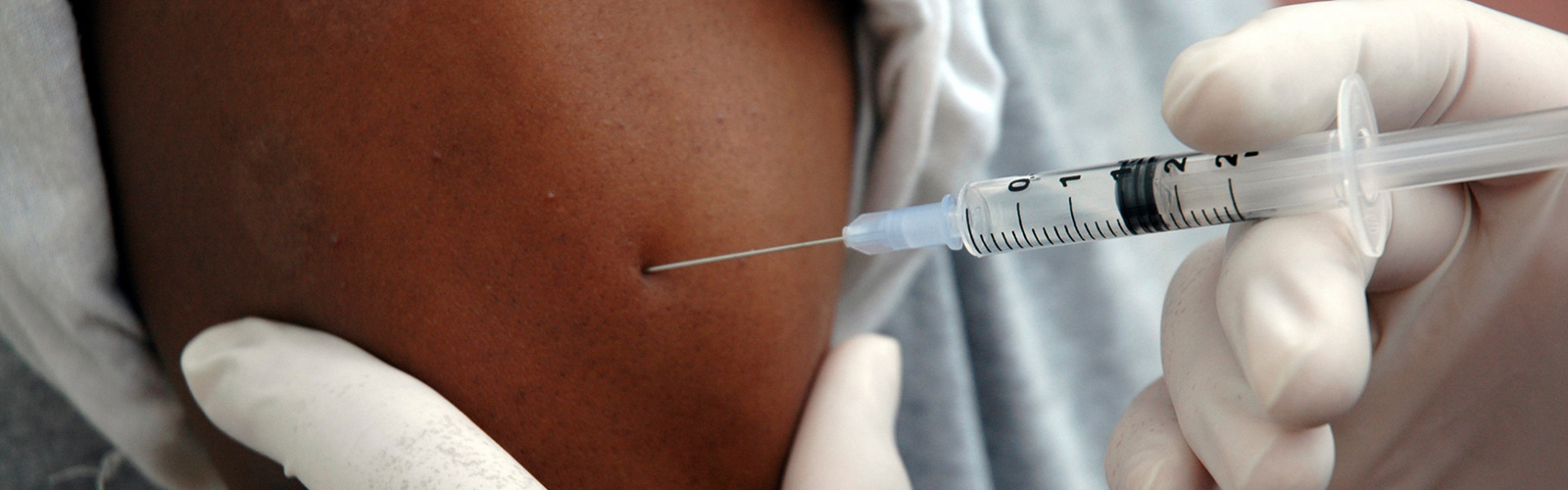
WASHINGTON, D.C. September 6 (C-Fam) In response to the results of a large-scale trial, the World Health Organization (WHO) has revised its recommendation for the use of the injectable contraceptive Depo Provera, removing warnings that it might be linked to increased risk of HIV infection.
When previous studies suggested such a link, the WHO faced pressure from activists to issue new guidance urging women at high risk of acquiring HIV not to use Depo. The WHO changed its eligibility criteria for such women from level 1 (no restrictions on its use) to level 2 (the advantages of its use are thought to outweigh the proven or suspected risks.) The new revision, issued on August 29th, reverses the previous change.
Many contraceptive proponents, such as FHI360, which has long championed injectables in particular, welcomed the revision. In a statement, they called for greater integration of HIV prevention services and family planning, and stated, “a woman’s risk of HIV acquisition should not restrict her contraceptive options.”
Others were skeptical. A group of over sixty experts and activists from twelve countries in Africa—where HIV risk remains greatest—wrote an open letter to the WHO when the results of the ECHO trial were announced, raising questions about its conclusions and requesting more time for deliberation before issuing any pronouncements. In particular, they questioned whether the study authors were justified in deciding that an increased risk of HIV of less than 50% was “not clinically relevant.”
Many of those expressing concerns were from South Africa, which is home to almost 90% of the study participants who contracted HIV during the trial period.
One signatory called the new WHO guidance “disturbing”: “For us it shows that they are prioritizing pharmaceutical companies over women’s priorities and voices.”
Another signatory explained that the guidance could have a large impact “as it has the power to determine, especially in low-income countries, the distribution of the drug in their national programs.”
Injectable contraceptives have been heavily promoted in Africa, particularly by the Gates Foundation, since they require little user activity and do not require removal, unlike intrauterine devices and implants.
Research from South Africa reveals that black women were particularly urged to use Depo, and that its popularity is driven by health workers’ recommendations, not user preference alone.
Apart from the debated link between Depo use and HIV infection, the injectable contraceptive has also been linked to other risks and side effects, which were not examined by the ECHO trial. The U.S. Food and Drug Administration has labeled it with a “black box warning” due to its link to bone density loss and recommends that the drug not be used for prolonged periods or by adolescents.
In its statement announcing the revised guidance, the WHO encouraged integrating family planning with HIV services and “include access to sexual and reproductive health services more broadly.” Global abortion groups have long called for the U.S. to integrate family planning and HIV services allowing them to be eligible to the $6B allocated to HIV/AIDs funding in comparison to the just $600M the U.S. directs to overseas family planning. It is this integration that led to the Trump administration expanding the Mexico City Policy beyond family planning to all global health funding.
View online at: https://c-fam.org/friday_fax/who-downgrades-warnings-on-depo-concerns-remain/
© 2024 C-Fam (Center for Family & Human Rights).
Permission granted for unlimited use. Credit required.
www.c-fam.org